Quality Policy
- Quality is the most important goal and the fundamental strategy for the success and growth of IT-Chem.
- We produce every products to meet the specifications and delivery requirements of IT Chem customers.
- The goal of IT-Chem's quality policy is to ensure stable product quality by continuously optimizing the quality system through passionate and well-trained members who actively and efficiently manage it. All products are developed, manufactured, tested, and shipped in accordance with ICH Q7A and Good Manufacturing Practice (GMP) guidelines (2022), achieving IT Chem's Vision.
- Through IT Chem's quality policy, we supply high-quality products reliably to meet customer expectations and secure cost savings in manufacturing and high-efficiency operations.
Quality Department
Quality (guaranteed) Manager
Quality Assurance Department
QA
RA
Quality Control Department
Analysis
RA
Microorganism
GMP SYSTEM
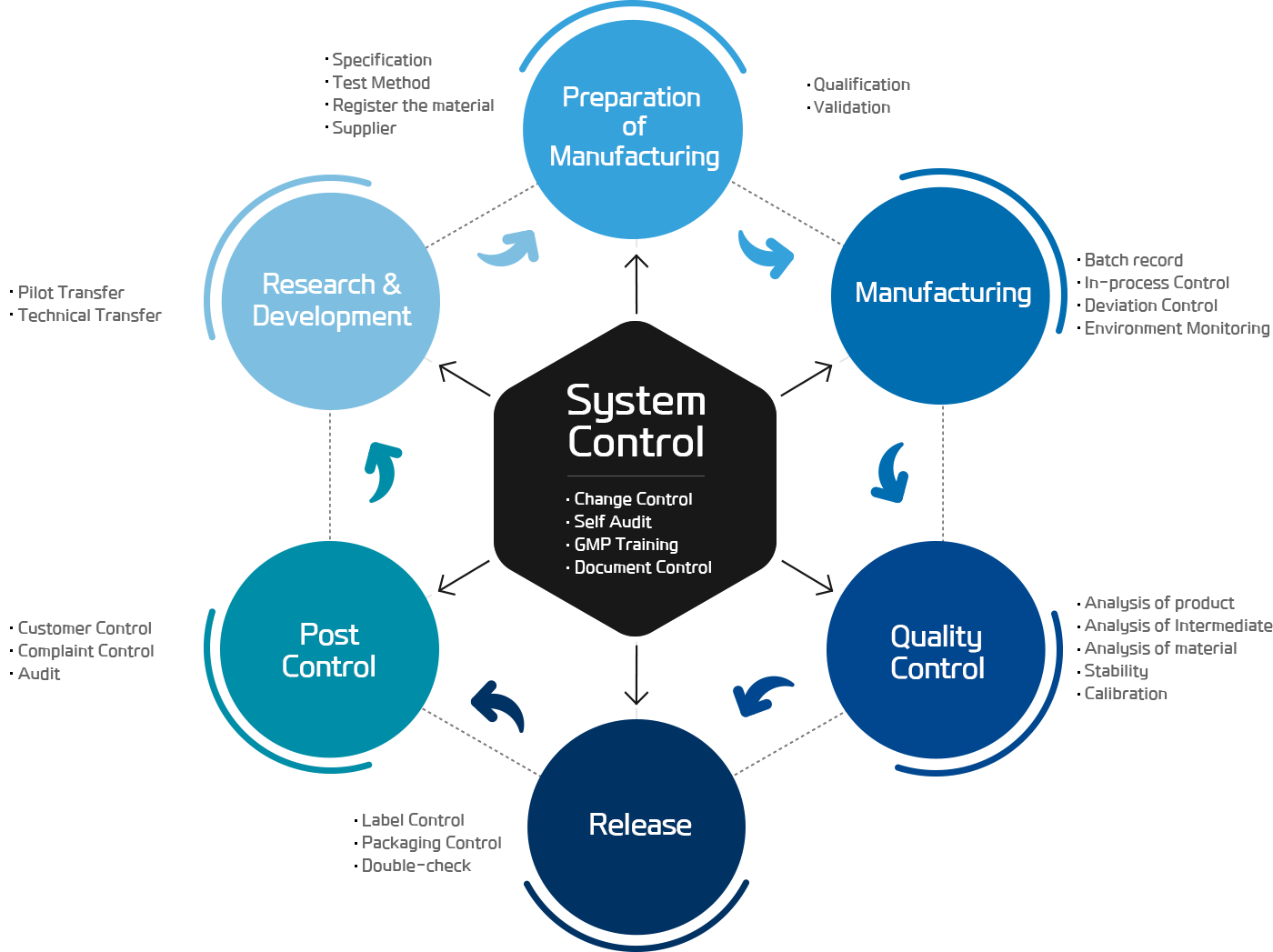
Quality Assurance
IT-Chem's quality assurance system is actively working in an organized and planned manner to ensure the quality meets customer expectations and building trust.
In 2015, we began registering active pharmaceutical ingredients through Korea's MFDS and quickly established an appropriate quality assurance system.
IT-Chem's quality assurance system is continuously improving through quality audits from numerous clients, including renowned finished product companies. Additionally, we are making efforts to register active pharmaceutical ingredients in foreign countries, including Japan's PMDA.
Quality assurance department, independent from the production, monitors deviations in production activities and quality control, possesses appropriate regulations for documenting investigations of these deviations, and has an appointed responsible manager.